Summary
In an international effort including Dr. Ana-Maria Orbai, M.D., M.H.S. from the Johns Hopkins Division of Rheumatology, patients and physicians came together to agree on what characteristics are critical to be measured in psoriatic arthritis clinical trials. The characteristics that are measured in clinical trials tells us how effective a drug is in treating psoriatic arthritis. For example, we currently record the number of tender and swollen joints in psoriatic arthritis, and an effective drug will reduce this number after treatment. But while joint inflammation is important to measure, it is not the only important outcome to monitor in psoriatic arthritis clinical trials, especially from the patients’ point of view. This project was critical in defining what outcomes are important for both patients and their doctors.
Why was this study done?
How was this study done?
The study consisted of multiple steps. Step 1: international focus groups with patients in 6 countries who told us how psoriatic arthritis affects their life. Step 2: systematic literature review of what has been measured to date in psoriatic arthritis clinical trials. Step 3: results from the first two steps were assembled in a list of outcomes (example fatigue, swollen joints, etc) which was sent to patients and physicians separately to vote on how important each outcome on the list is to them. Step 4: results from step 3 were discussed in a face-to-face meeting of 12 patients and physicians with a neutral moderator who used the “nominal group technique” to ensure that the voice of each participant was taken into consideration. At the end of this phase, 10 critical outcomes were agreed on by both patients and physicians and a few additional outcomes were considered important but not mandatory to measure. Step 5: The 10 outcomes were sent for voting again separately to patients and physicians. At the end of this phase, results were presented at the international conference Outcome Measures in Rheumatology for voting and endorsement.
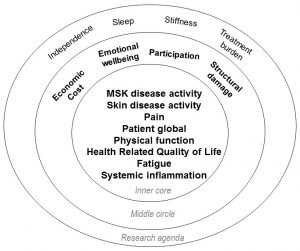
What were the major findings?
As shown on the right, this study identified eight outcomes that were voted as critical to be measured in every clinical trial (inner core), four voted important but not mandatory for every clinical trial (middle core), and four others that were deemed important but require further study (research agenda). From this, we learned that the perspectives of patients and physicians are different when considering the importance of outcomes in psoriatic arthritis. Both perspectives are important to consider in order to select a truly relevant set of outcomes which best measures the impact of a therapy. In addition, we learned that patients value improvements in fatigue, social participation, and emotional well-being in addition to improvements valued by both patients and physicians including pain, joint swelling, skin disease, inflammation, physical function and damage.
What is the impact of this work?
Clinical trials measuring these outcomes will include these results in the description of drug effectiveness for treating the symptoms of psoriatic arthritis that are important to patients as well as physicians. This will allow a better discussion of therapy options between patients and their physicians and enable personalized treatment decisions.
This research was supported by:
The Rheumatic Disease Research Core Center at the Johns Hopkins Division of Rheumatology and the P30 award sponsored by the National Institutes of Health.
Link to original research article:
International patient and physician consensus on a psoriatic arthritis core outcome set for clinical trials. Orbai AM, de Wit M, Mease P, Shea JA, Gossec L, Leung YY, Tillett W, Elmamoun M, Callis Duffin K, Campbell W, Christensen R, Coates L, Dures E, Eder L, FitzGerald O, Gladman D, Goel N, Grieb SD, Hewlett S, Hoejgaard P, Kalyoncu U, Lindsay C, McHugh N, Shea B, Steinkoenig I, Strand V, Ogdie A. Ann Rheum Dis. 2016 Sep 9.